Disclaimer and contact information
About surgical techniques:
Proper surgical procedures and techniques are the responsibility of the medical professional. The guidelines contained herein are furnished for information purposes only. Each surgeon must evaluate the appropriateness of the procedures based on his or her personal medical training and experience. Prior to use of any Merete systems, the surgeon should refer to the product package insert for complete warnings, precautions, indications, contraindications and adverse effects. Package inserts are also available by contacting Merete Technologies, Inc.
About E-IFUs:
If you have any further questions about the sterilization processes after reading our instructions, contact us at 630-869-1091 or service@merete-medical.com.
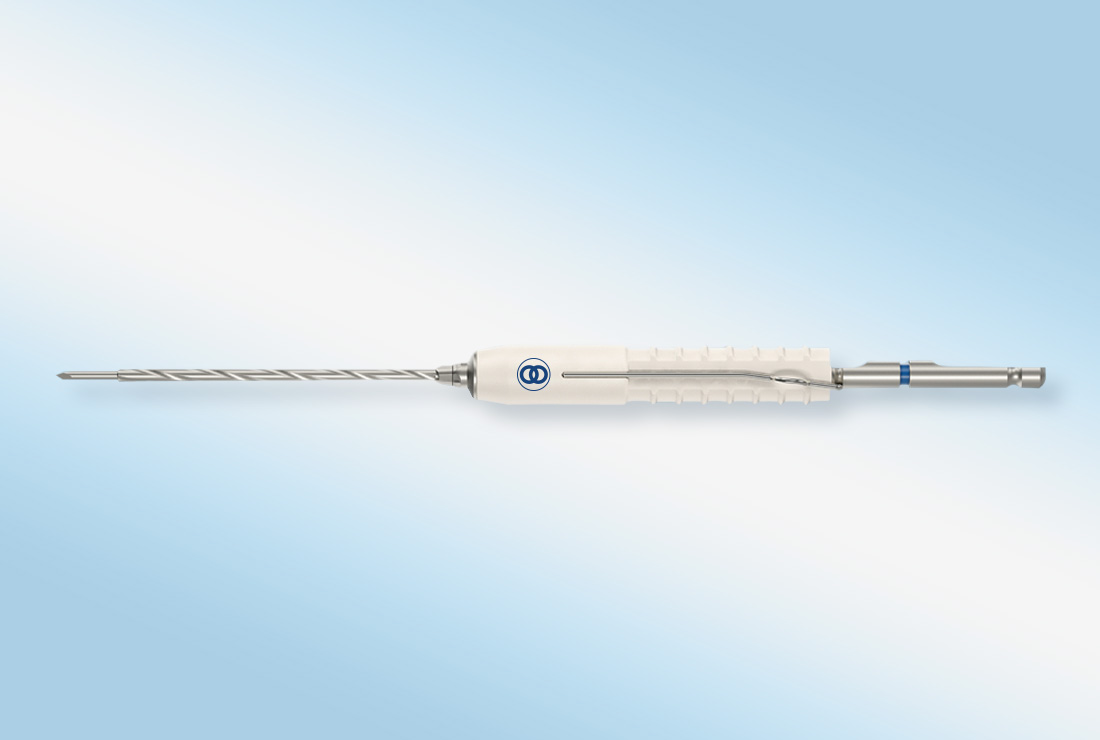
Mecron® Cannulated Screws
Re-introducing the proven Mecron® Cannulated Screw, re-designed for today’s foot and ankle surgeons…Merete® founder Emmanuel Anapliotis developed and launched the proven cannulated orthopedic screw in the early 1970s. CEO Alexia Anapliotis has updated her father’s historic Mecron® headed and headless screws to exceed the expectations of 21ST century foot and ankle surgeons.
The Mecron® Cannulated Screw consists of headed and headless compression screws made from titanium alloy. The screws are available in diameters from 2.0 mm through 4.0 mm and overall lengths from 8 mm through 50 mm. The Mecron® Screw is designed to provide optimal compression, precise tracking and has highly-engineered reverse cutting flutes for easy removal.
Technical Specifications
- Made of titanium alloy (Ti6AI4V ELI, ISO 5832-3, ASTM F136)
- Available as headed and headless compression screws
- Self-drilling/self-tapping
- With short, full and long threads
- Cannulated
FEATURES
- Headless screws are furnished with a tapered neck to ensure pre-compression before and prevent cracks while the proximal thread engages cortical bone
- Reduced insertion forces lead to enhance surgical ease-of-use
- Optimal pull-out strength and improved compression of bone fragments while the thread design lowers the risk of stripping
- Washers are available to prevent plunging of headed screws in soft-bone
- Reverse cutting-flutes facilitate removal
- Color-coded instrumentation across all screw diameters to quickly and easily identify drills, countersinks, tissue protectors and screw drivers
- Self-locking screw drivers hold the screw in place during insertion and a screw extractor is available for explantation, if needed
Indications
- Bone Reconstruction
- Osteotomy
- Arthrodesis
- Joint Fusion
- Ligament Fixation
- Fracture Repair
- Fracture Fixation appropriate for the size of the device