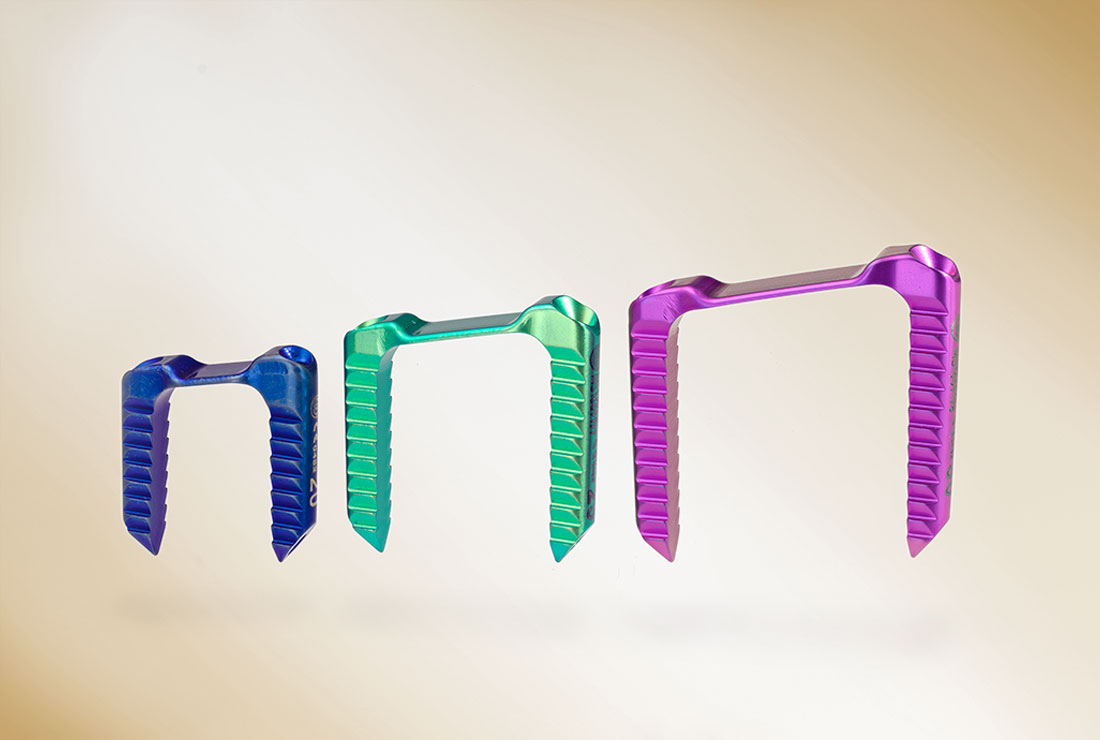Disclaimer and contact information
About surgical techniques:
Proper surgical procedures and techniques are the responsibility of the medical professional. The guidelines contained herein are furnished for information purposes only. Each surgeon must evaluate the appropriateness of the procedures based on his or her personal medical training and experience. Prior to use of any Merete systems, the surgeon should refer to the product package insert for complete warnings, precautions, indications, contraindications and adverse effects. Package inserts are also available by contacting Merete Technologies, Inc.
About E-IFUs:
If you have any further questions about the sterilization processes after reading our instructions, contact us at 630-869-1091 or service@merete-medical.com.
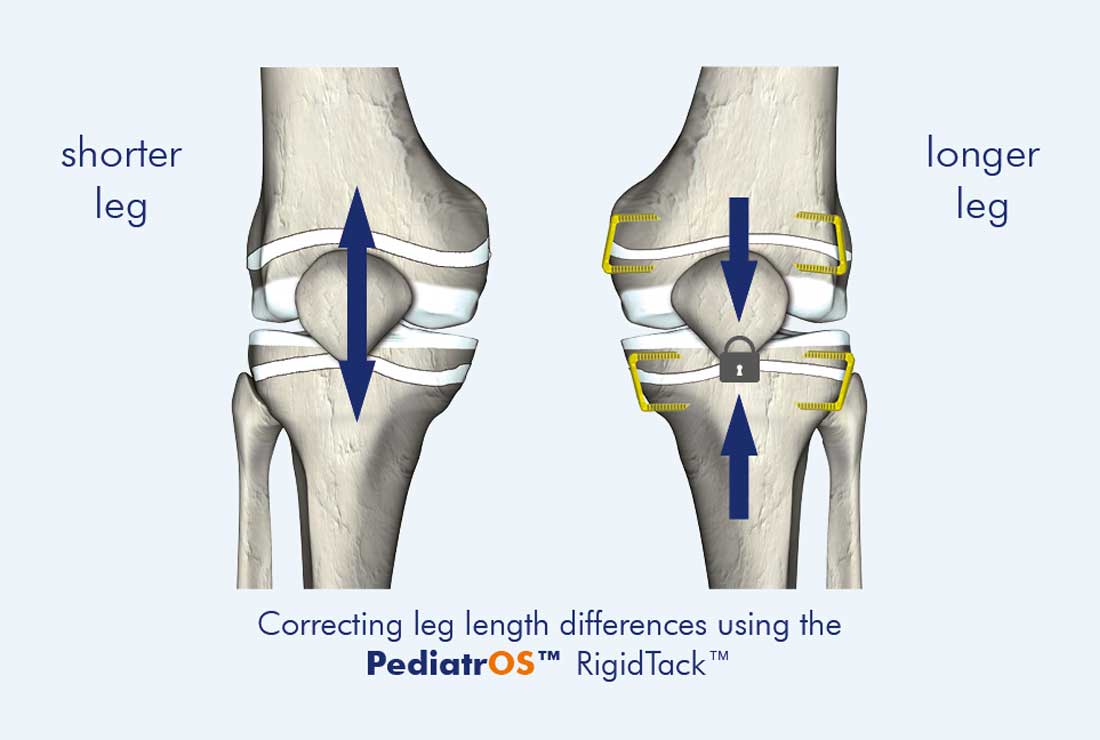
PediatrOS™ RigidTack™
Growth arrest for leg length differences
To ensure that the staples will behave rigidly and mechanically, their central areas are reinforced especially well, allowing stable, precise temporary epiphysiodesis. The trapezoidal design of the PediatrOS™ RigidTack™ staple is closely aligned to the anatomy of the femur and tibia. Cannulated legs allow accurate placement using K-wires. Low fluoroscopy times are another advantage. The PediatrOS™ RigidTack™ is the only growth arrest implant on the market specifically developed and approved for correcting leg length differences in children and adolescents.
Technical Data
- Trapezoidal design
- 3 sizes: 20 mm, 25 mm and 30 mm
- Barbed staple legs
- Cannulated staple legs for guided insertion over 1.6 mm K-wires and optimal growth arrest through parallel staple legs aligned with the physis
FEATURES
- First anatomically shaped titanium implant designed to correct leg length differences
- Reinforce staple bridge achieving physis compression that is equivalent to three Blount staples. Reduced complication rates by preventing staple dislocation or migration through barbed staples legs that safely anchor the implant in bone. Secondary frontal plane deformities such as volcano effects occurring with two-hole plates or Varus deformities induced through Blount staple dislocations are less prevalent
- Supplied sterile
- Minimal invasive surgery and reduced overall OR-time. Achieved by reducing the number of implants from two to three Blount staples to a single device per epiphysiodesis site
- Ideal biomechanical alignment
- Fluoroscopy time reduction of ~65 % compared to other stapling procedures. Consequently, reduced x-ray exposure for patients
- Alignment template ensures precise placement
- Easy to remove using 2.0 mm K-wire with threaded tip
- ONE set of instruments for two indications: PediatrOS™ FlexTack™ and PediatrOS™ RigidTack™
- Immediate weight-bearing possible.
Indications
Specific pediatric conditions/diseases for which the devices will be indicated include leg length discrepancies
Radiographs (Coming Soon)