OsteoBridge™ knee arthrodesis system · knee arthrodesis
OsteoBridge™ knee arthrodesis system · knee arthrodesis
Disclaimer and contact information
About surgical techniques:
Proper surgical procedures and techniques are the responsibility of the medical professional. The guidelines contained herein are furnished for information purposes only. Each surgeon must evaluate the appropriateness of the procedures based on his or her personal medical training and experience. Prior to use of any Merete systems, the surgeon should refer to the product package insert for complete warnings, precautions, indications, contraindications and adverse effects. Package inserts are also available by contacting Merete Technologies, Inc.
About E-IFUs:
If you have any further questions about the sterilization processes after reading our instructions, contact us at 630-869-1091 or service@merete-medical.com.
OP-Video Surgical Technique E-IFU
OsteoBridge™ IKA – Knee Arthrodesis System
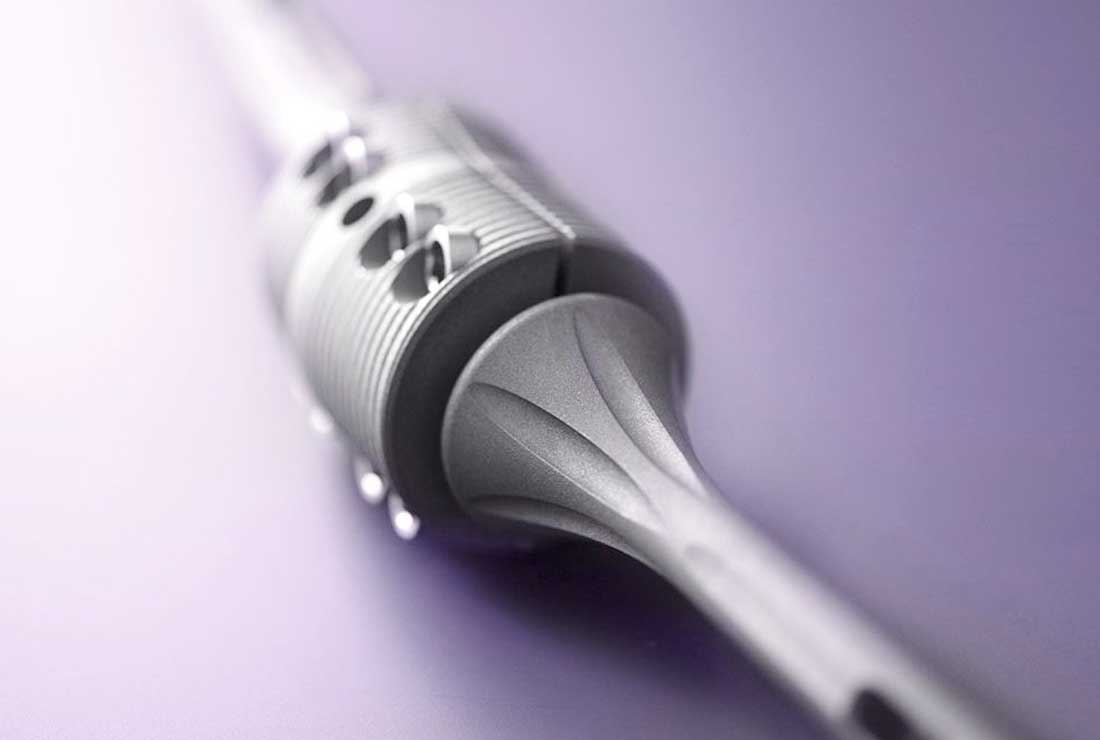
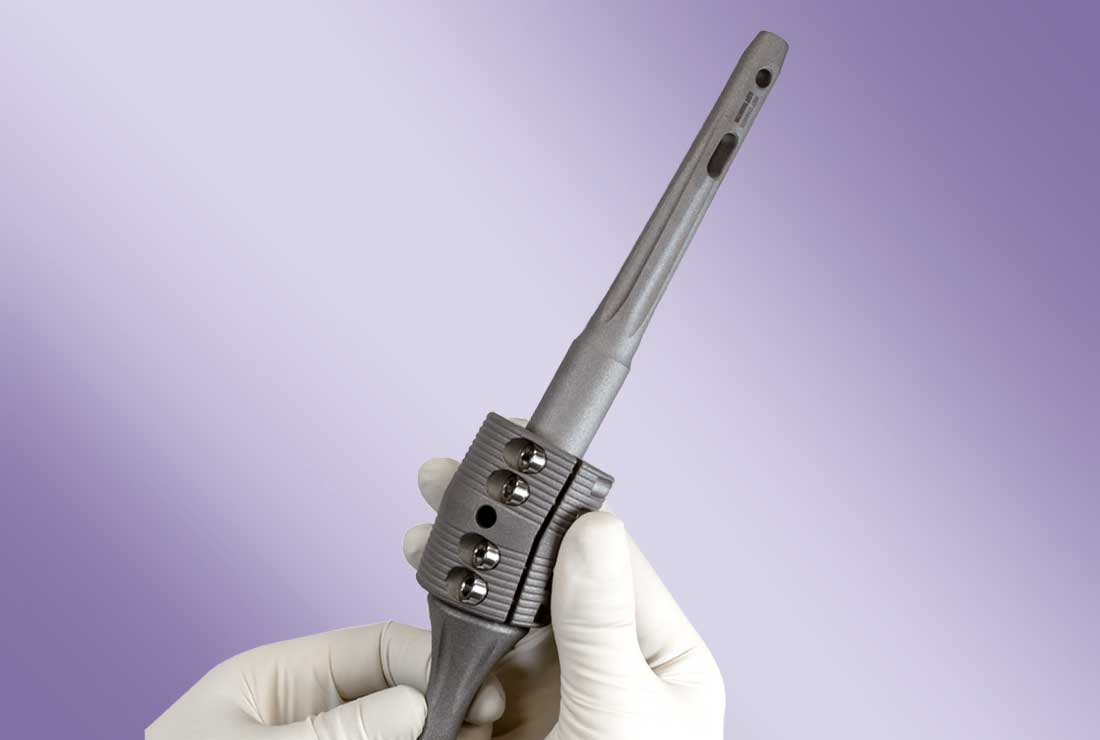
The OsteoBridge™ IKA Intramedullary Knee Arthrodesis system serves to fuse the knee joint. The use of the implant is also possible to bridge long bone defects and for resection of the metaphyseal area of the femur and tibia. OsteoBridge™ IKA knee arthrodesis allows cementless or cemented restoration. For the fusion of the knee joint with optimal anatomical adaptation and as an alternative to the treatment with plates or after plate fractures, the modular OsteoBridge™ system is a simple and stable solution for limb preservation.
Technical Specifications
The OsteoBridge™ IKA system spacer is available with a fixed 10° angle, a standard length of 50 mm and a diameter of 40 mm. All nail design variations and sizes can be used with the IKA system spacer. Straight nails with a collar are used in revision procedures to replace previous prostheses. Intramedullary nails are available in lengths from 130 mm through 300 mm and in diameters from 10 mm through 20 mm.
Features
- System modularity ensures flexibility and enables surgeons to use any nail size with the included IKA spacer, and thus, to adapt to the particular bone defect
- Cemented and cementless implantation
- Primary stability is achieved by pressfit and partly tapered, wedge-shaped nail designs
- Secure secondary stability is achieved with biological integration
- Slight extension/flexion and valgus/varus extremity adjustments can be made with the positioning of the spacer
- Possibility of immediate load-bearing of the affected extremity and mobilization of the patient
- All components are made of Ti6Al4V ELI
- Limb salvage system as alternative to an amputation
- Fast surgery with optimal ease-of-use
INDICATIONS
- Irretrievably failed total knee arthroplasty
- Limb salvage
- Oncology surgery
- Any other condition where there is little soft tissue or bony tissue available for support and arthrodesis is the treatment of choice
Radiographs



