Disclaimer and contact information
About surgical techniques:
Proper surgical procedures and techniques are the responsibility of the medical professional. The guidelines contained herein are furnished for information purposes only. Each surgeon must evaluate the appropriateness of the procedures based on his or her personal medical training and experience. Prior to use of any Merete systems, the surgeon should refer to the product package insert for complete warnings, precautions, indications, contraindications and adverse effects. Package inserts are also available by contacting Merete Technologies, Inc.
About E-IFUs:
If you have any further questions about the sterilization processes after reading our instructions, contact us at 630-869-1091 or service@merete-medical.com.
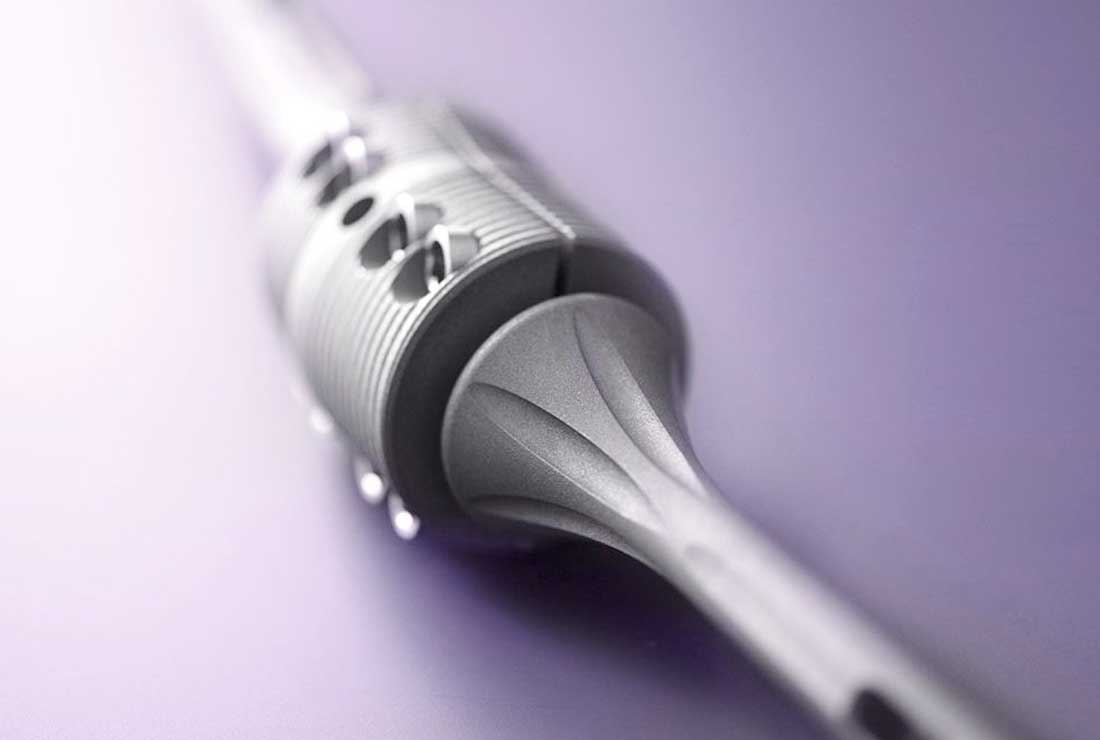
OsteoBridge™ IDSF – Intramedullary
Diaphyseal Segmental Defect Fixation System
OsteoBridge™ IDSF is a modular diaphyseal implant used for long term stabilization of segmental bone defects in humerus, femur and tibia of oncology patients. The modular endoprosthesis spacer consists of a spacer(s), that fixates two intramedullary nails and bridges the defect after resection.
Technical Specifications
The modular OsteoBridge™ IDSF endoprosthesis consists of two semi-circular shells assembled and fixated with eight screws over two intramedullary nails that extend past the osteotomy planes and into a spacer. The intramedullary nails can additionally be interlocked distally. The spacers and the nails are available in different dimensions for flexibility across defect sizes from 40 to 140 mm. The outer spacer diameters are 20 mm (humerus), 25 mm (tibia) and 34 mm (femur).
Spacers are available in lengths of 40 mm, 50 mm, 60 mm and 70 mm. If a longer defect is to be bridged, two or more spacers may to be combined with spacer connectors. Intramedullary nails are available in lengths from 60 mm through 200 mm and in diameters from 7 mm through 20 mm.
Features
- High modularity for optimal adaptation to the bone. A force fit between nail and spacer facilitates continuous intra-operative rotation until the screws are tightened down. Subsequently, rotational stability is preserved post-operatively.
- The OsteoBridge™ IDSF System allows for bridging of defects from 40 mm to 140 mm in 10 mm steps
- Fast surgery with optimal ease-of-use
- Possibility of immediate load-bearing of the affected extremity and mobilization of the patient
- Nails and spacers can be rotated and implanted in any orientation
- Long term stability due to biological integration
- Surgeon may customize modular implant to exact bone resection
- Cementless and cemented implantation possible
- All components are made of Ti6Al4V ELI
INDICATIONS
For the management of segmental diaphyseal bone loss of either humerus, tibia or femur in oncology patients secondary to radical bone loss and/or resection due to tumors. The intramedullary rods can be fixed with interlocking screw without or with bone cement.
Radiographs