Disclaimer and contact information
About surgical techniques:
Proper surgical procedures and techniques are the responsibility of the medical professional. The guidelines contained herein are furnished for information purposes only. Each surgeon must evaluate the appropriateness of the procedures based on his or her personal medical training and experience. Prior to use of any Merete systems, the surgeon should refer to the product package insert for complete warnings, precautions, indications, contraindications and adverse effects. Package inserts are also available by contacting Merete Technologies, Inc.
About E-IFUs:
If you have any further questions about the sterilization processes after reading our instructions, contact us at 630-869-1091 or service@merete-medical.com.
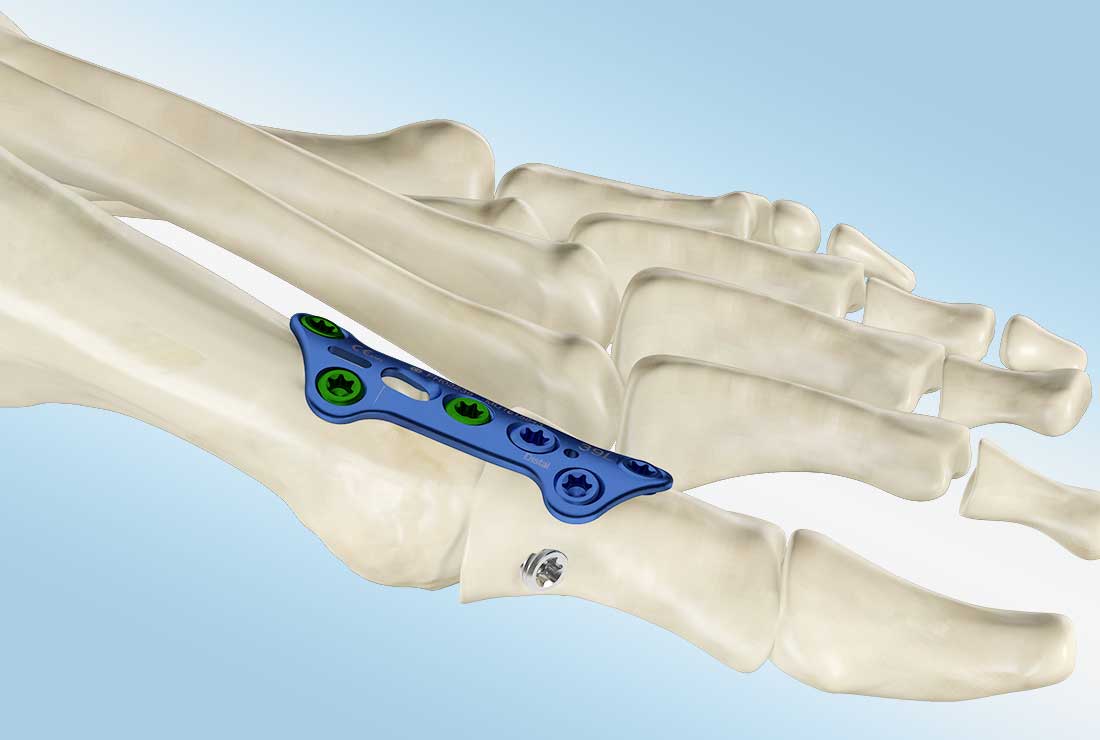
MetaFix™ MTP
The Merete® MTP-Fusion Plate is applied in Hallux Rigidus interventions or revision procedures for failed arthroplasties of the first metatarsophalangeal joint (MTP1). MTP1 joints are fused and fixated with a rigid locking plate construct and compression screws.
Technical Specifications
- Plates are pre-bent with 5° of dorsiflexion and 5° of valgus
- A proximal compression long-hole slot compresses the fusion site during the insertion of a headed plate compression screw that slides along a ramp in the plate to compress the fragments underneath
- The Plate and screws made of titanium alloy (Ti6AI4V ELI, ISO 5832-3, ASTM F136)
- Low Profile, anatomically pre-formed locking plate with 5° of dorsiflexion and a 5° valgus angle for left and right feet
- Used with 3.0 mm and 3.5 mm MetaFix ™ LS locking screws
- K-wire holes in the plate facilitate temporary fixation for locking screw insertion
FEATURES
- Plate lengths 39 and 52 mm available for left and right feet
- 3.0 mm and 3.5 mm MetaFix® LS locking screws combined with cannulated 3.0 mm plate compression screws (PCS) and cannulated 3.0 mm headed compression screws (HCS)
- Pre-formed 5° dorsiflexion and 5°valgus angles
- Long-hole compression slot proximally for additional compression of the fusion site
Indications
Fixation of fresh fractures, revision procedures, joint fusion, and reconstruction of small bones of the hand, feet, wrist, ankles, fingers, and toes.